The United Kingdom’s (UK) Health & Safety Executive (HSE) has begun a public consultation on a legislative proposal to reform the Great Britain (GB) Classification, Labelling and Packaging (CLP) Regulation. HSE notes that GB CLP Article 37 links mandatory classification and labeling (MCL) in GB to evaluation activity in the European Union’s (EU) harmonized classification and labeling (CLH) system by creating a statutory obligation to consider the European Chemicals Agency’s (ECHA) Committee for Risk Assessment’s (RAC) opinions on harmonized classification proposals made under the EU CLP. This consideration is required “even for those which consider substances or hazard classes not authorised for use in GB.” The requirement to consider RAC opinions that are not relevant to GB adds additional burdens for the regulator and is exacerbated by the recent EU CLP revisions. The European Commission (EC) introduced six new hazard classes into the EU CLP, and these classes are prioritized for consideration under the EU CLH system. HSE states that this revision to the EU CLP “will result in a greater proportion of RAC opinions featuring non-GB CLP hazard classes.” HSE notes that the statutory timelines in Article 37 of the GB CLP are currently triggered when a RAC opinion is published, “requiring evaluations to be sequenced by the RAC opinion publication date determined for the EU.” According to HSE, the current timelines restrict its ability to prioritize its GB MCL evaluation work appropriately and to provide regulatory clarity to a timescale dictated by relevance to the GB market.
HSE states that it believes amending the GB CLP is necessary to provide greater certainty for duty holders and to ensure that future GB MCL evaluation activity can be delivered “predictably and sustainably.” HSE proposes to consolidate GB CLP Articles 37 and 37A into one procedure under which MCL proposals would be evaluated, thereby simplifying the process for substance and mixture classification in GB. HSE notes that the consolidated procedure would include a fast-track evaluation pathway for assessing classification proposals from territories that adopt the United Nations (UN) Globally Harmonized System of Classification and Labelling of Chemicals (GHS).and have a transparent classification process. Classification proposals from jurisdictions that do not adopt the UN GHS and do not have a transparent classification process would be evaluated under a full process, similar to that currently described in Article 37A. HSE provides a flowchart showing a possible route to fast-track evaluation for assessing classification proposals from UN GHS adopting territories that have a transparent classification process:
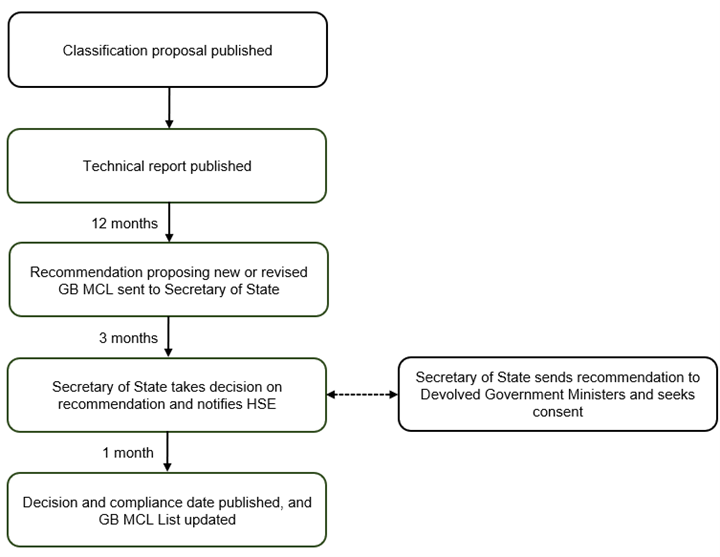
HSE also proposes to omit from the consolidated procedure the legal requirement for it to send a copy of its ministerial recommendation to devolved government (DG) ministers. According to HSE, “[t]his would reduce the administrative burdens arising from this aspect of delivery of the GB MCL system and ensure that resource is used proportionately.” HSE notes that it “remains committed to the evaluation of classification proposals that focus on carcinogenic, mutagenic, reproductive toxic and respiratory sensitising hazards” and states that these proposals would be prioritized for fast-track evaluation where they originate from the EU. Comments are due August 18, 2025.