Congress Declines to Extend HDHP First-Dollar Telehealth Coverage Relief
After Congress declined to extend certain relief allowing first-dollar coverage of telehealth services by high-deductible health plans (HDHPs), health plan sponsors may need to make immediate changes to preserve employees’ health savings account (HSA) eligibility.
Quick Hits
Due to the expiration of certain relief that allowed pre-deductible coverage of telehealth, employers offering HDHPs with first-dollar telehealth coverage may need to amend their plans by January 1, 2025 (for calendar year plans) to ensure employees remain eligible to contribute to their HSAs.
In connection with this change, plan sponsors may also need to update their HDHP participant communications to reflect changes in cost sharing for telehealth services.
As mentioned in our December 3, 2024, article on HDHP plan amendments, the CARES Act of 2020, which was extended through the Consolidated Appropriations Act, 2023, allowed, but did not require, HDHPs to provide first-dollar coverage of telehealth without negatively affecting participants’ HSA eligibility. The extension expired at the end of the 2024 plan year (December 31, 2024, for calendar year plans), and Congress’s year-end spending bill, the American Relief Act, 2025, did not include an extension of the HDHP telehealth relief.
Accordingly, an employer that provides HDHP health plan coverage will need to amend its HDHP if it includes first-dollar telehealth coverage. Since the prior relief was not extended, individuals who are covered by an HDHP that covers telehealth services before the deductible will not be eligible to contribute to an HSA for some or all of 2025.
Effective January 1, 2025 (for a calendar year plan), to preserve employees’ HSA eligibility, an HDHP that covers telehealth services may not cover such services until the employee has met the annual deductible. Employers with non–calendar year plans will have until the end of the plan year that began in 2024 to make the change. In either case, employers will want to confirm that their plan documents, summary plan descriptions, and summaries of benefits and coverage are updated to reflect any changes to participant cost sharing for telehealth services.
Second Circuit Revives New York Reproductive Health Bias Law’s Notice Requirement for Employee Handbooks
On January 2, 2024, the U.S. Court of Appeals for the Second Circuit reinstated the New York Reproductive Health Bias Law’s requirement that New York State employers include a notice in their employee handbooks regarding the law’s prohibition on discrimination and retaliation based on employees’ reproductive health care choices.
Quick Hits
The Second Circuit has revived a requirement that New York employers include in employee handbooks a notice informing employees of their right to be free from discrimination or retaliation based on their [the employees’] or their dependents’ reproductive health decisions.
The ruling also revived a First Amendment challenge by religious organizations to New York’s Reproductive Health Bias Law (New York Labor Law Section 203-e), impacting how employers may address expressive association claims in the employment context.
In CompassCare v. Hochul, three religious groups—CompassCare, the National Institute of Family and Life Advocates (NIFLA), and First Bible Baptist Church—challenged the constitutionality of New York Labor Law Section 203-e, which went into effect in November 2019.
The law prohibits employers from accessing personal information regarding employees’ or their dependents’ reproductive health decision making without the employees’ “prior informed affirmative written consent.” The law also prohibits employers from discriminating or retaliating against employees based on their reproductive health decisions, “including, but not limited to, a decision to use or access a particular drug, device, or medical service.” Importantly, the law included a notice provision requiring employers to inform employees of their rights and remedies under the law in employee handbooks.
On March 29, 2022, the U.S. District Court for the Northern District of New York entered a permanent injunction blocking the State of New York from enforcing the requirement that employers that issue employee handbooks “include in the handbook notice of employee rights and remedies under [Section 203-e].” The district court found that the notice provision of Section 203-e violated the First Amendment because it compelled speech that was contrary to the religious organizations’ religious beliefs as they related to reproductive choices.
The Second Circuit reversed that permanent injunction, finding the notice requirement “a content-based regulation of speech” that “is subject to … rational basis review.” Under that review, the Second Circuit found that the notice requirement did “not interfere with [the] [p]laintiffs’ greater message and mission” and that “the required disclosure of the existence and basic nature of an otherwise-valid statute” was a simple expression of employee rights, similar to many other required employment rights notices and postings.
Additionally, the Second Circuit remanded the case to the district court for reconsideration in light of the Second Circuit’s 2023 decision in Slattery v. Hochul, which held that an employer may have an associational rights claim if the law “forces [the employer] to employ individuals who act or have acted against the very mission of its organization.” (Emphasis in the original.)
The Second Circuit stated that to sustain such a claim, an employer must show that it does not simply hold particular views or interests but that an association threatens the “very mission” of the employer “in the context of a specific employment decision.” This showing would be based on an assessment of whether (1) a position at issue is client-facing or involves expressing the particular views of the employer, and (2) the conduct or specific attribute of an employee “renders the employment of that person, in that position, a threat to the employer’s mission,” the court stated.
Next Steps
As a result of this ruling, New York employers must immediately comply with the notice provision of Section 203-e. Thus, employers with New York employees that issue employee handbooks must include a notification to employees of their rights and remedies under Section 203-e in their employee handbooks or in an addendum containing New York–specific employment policies.
This requirement includes informing employees of their rights to make reproductive health decisions and not be discriminated against or retaliated against for such decisions.
With respect to the expressive association claim, employers, particularly those with specific missions or religious affiliations, may have grounds to challenge laws that they believe force them to employ individuals whose actions conflict with their organizational missions. However, such claims must be specific and demonstrate how the law threatens the organization’s mission in the context of particular employment decisions.
HHS-OCR’s Proposed Rule and HIPAA Security Risk Assessment
On December 27, 2024, in the midst of the holiday season, the U.S. Department of Health and Human Services (HHS) deployed a proposed rule that would significantly modify the Health Insurance Portability and Accountability Act (HIPAA) Security Rule. Specifically, the proposed new rule includes express requirements for Covered Entities when conducting a Security Risk Assessment (SRA).
New requirements would include a written assessment that contains, among other things:
A review of the technology asset inventory and network map
Identification of all reasonably anticipated threats to the confidentiality, integrity, and availability of ePHI
Identification of potential vulnerabilities and predisposing conditions to the regulated entity’s relevant electronic information systems
An assessment of the risk level for each identified threat and vulnerability, based on the likelihood that each identified threat will exploit the identified vulnerabilities.
Notably, while the “new” requirements have yet to be finalized or take effect, HHS’s Office of Civil Rights (HHS-OCR) has already begun to enforce these requirements on Covered Entities including the imposition of fines and penalties against Covered Entities whose failure to implement the proposed requirements result in a data breach affecting its patients’ protected health information (PHI).
For some time, HHS-OCR has acknowledged that the HIPAA Security Rule does not prescribe a specific risk analysis methodology, and it has recognized that methods of conducting a SRA will vary depending on the size, complexity, and capabilities of the organization. Further, HHS-OCR Guidance on Risk Analysis does not endorse or recommend any particular risk analysis or risk management model. While HHS-OCR provides a free proprietary tool for small to medium-size organizations to use when conducting a SRA, its product contains a disclaimer that use of the tool does not guarantee compliance with federal, state, or local laws.
Covered entities are therefore left to their own devices in discerning what methodologies and management models are appropriate for their organization when conducting a SRA. At the same time, the adopted methodology that an organization chooses may not be considered insufficient under HHS-OCR’s undisclosed standards. A Covered Entity with no SRA or an insufficient SRA may face significant fines and penalties in the event they are subject to a data breach and subsequent HIPAA compliance audit.
While Covered Entities may turn to third-party vendors that market themselves as specialists in providing HIPAA compliance services, including conducting SRAs, there is no guarantee this will satisfy the requirements under HIPAA. Recently, HHS-OCR has regarded SRAs performed by these vendors as deficient without providing any specific guidance to the Covered Entity as to exactly what aspects of their SRA were noncompliant with HIPAA.
This conundrum has recently dismayed a number of Covered Entities that are now facing fines and penalties in light of HHS-OCR’s recent HIPAA Security Risk Assessment enforcement initiative, which it has relentlessly pursued since October of 2024. It’s not yet clear whether the proposed requirements will make compliance with HIPAA’s Security Rule easier or create further confusion.
This Week in 340B: January 7 – 13, 2025
Find this week’s updates on 340B litigation to help you stay in the know on how 340B cases are developing across the country. Each week we comb through the dockets of more than 50 340B cases to provide you with a quick summary of relevant updates from the prior week in this industry-shaping body of litigation.
Issues at Stake: Contract Pharmacy; Other
In two appealed cases challenging a proposed Louisiana law governing contract pharmacy arrangements, the appellants filed their opening brief.
In a breach of contract case related to the Medicare 340B cuts, the court terminated the action without prejudice.
Matt David, associate in McDermott’s Los Angeles office, also contributed to this blog post.
December 2024 Bounty Hunter Plaintiff Claims
California’s Proposition 65 (“Prop. 65”), the Safe Drinking Water and Toxic Enforcement Act of 1986, requires, among other things, sellers of products to provide a “clear and reasonable warning” if use of the product results in a knowing and intentional exposure to one of more than 900 different chemicals “known to the State of California” to cause cancer or reproductive toxicity, which are included on The Proposition 65 List. For additional background information, see the Special Focus article, California’s Proposition 65: A Regulatory Conundrum.
Because Prop. 65 permits enforcement of the law by private individuals (the so-called bounty hunter provision), this section of the statute has long been a source of significant claims and litigation in California. It has also gone a long way in helping to create a plaintiff’s bar that specializes in such lawsuits. This is because the statute allows recovery of attorney’s fees, in addition to the imposition of civil penalties as high as $2,500 per day per violation. Thus, the costs of litigation and settlement can be substantial.
The purpose of Keller and Heckman’s latest publication, Prop 65 Pulse, is to provide our readers with an idea of the ongoing trends in bounty hunter activity.
In December of 2024, product manufacturers, distributors, and retailers were the targets of 394 new Notices of Violation (“Notices”) and amended Notices, alleging a violation of Prop. 65 for failure to provide a warning for their products. This was based on the alleged presence of the following chemicals in these products. Noteworthy trends and categories from Notices sent in December 2024 are excerpted and discussed below. A complete list of Notices sent in December 2024 can be found on the California Attorney General’s website, located here: 60-Day Notice Search.
Food and Drug
Product Category
Notice(s)
Alleged Chemicals
Fruits, Vegetables, and Mushrooms: Notices include farro porcini mushrooms, chopped spinach, capers, chili mango, flavored sunflower seeds, shiitake mushrooms, kale chips, flax seeds, artichoke quarters in brine, moringa, dried apricot, madras lentils, cactus chips, bamboo shoots, and stuffed manzanilla olives
38 Notices
Lead and Lead Compounds, and Cadmium and Cadmium Compounds
Prepared Foods: Notices include soup bowls, noodle bowls, salt & vinegar potato chips, bundt cake mix, flatbread mix, granola bars, crackers, nut butter, vegetable biryani, vegan chips, mushroom ravioli, gluten-free tortilla wraps, and plant-based ground meat
36 Notices
Lead and Lead Compounds, Cadmium, and Mercury
Seafood: Notices include Alaska pink salmon, tuna salad, mackerel in olive oil, sardines, seasoned squid, dried seaweed, fried anchovy, dried mackerel, ground shrimp, dried sea mustard seaweed, raw seaweed, and shrimp paste
32 Notices
Lead and Lead Compounds, Cadmium and Cadmium Compounds, and Mercury
Dietary Supplements: Notices include plant-based protein shakes, green powder superfood, greens, protein powder, electrolyte formula beverages, pre-workout beverages, ginkgo biloba powder and tea, and spirulina powder
26 Notices
Cadmium, Lead and Lead Compounds, Mercury and Mercury Compounds, and Perfluorooctanoic Acid (PFOA)
THC-containing Products: Notices include gummies, chocolates, soft gels, flavored beverages, and candies
13 Notices
Delta-9-tetrahydrocannabinol
Sauces: Notices include red mole, aged balsamic vinegar, sundried tomato paste, and basil pesto sauce
4 Notices
Lead and Lead Compounds
Packaged Liquids: Notices include vegetable stock and fruit-flavored beverages, and canned coconut water
4 Notices
Perfluorononanoic Acid (PFNA) and its salts, Perfluorooctanoic Acid (PFOA), and Bisphenol A (BPA)
Cosmetics and Personal Care
Product Category
Notice(s)
Alleged Chemicals
Personal Care Items: Notices include hair color, aloe vera lotions, skin toners, spot treatments, face masks, vitamin C serum, enzyme scrub, body cleaners, eye serums and creams, hair color treatments, hair gels, body wash and foaming cleansers, pain relief cream, body glow, and squirt blood
66 Notices
Diethanolamine
Cosmetics: Notices include mascara, cream makeup, matte lipstick, eyeliner pens, concealers, face primer, and cake makeup
36 Notices
Diethanolamine
Personal Care Products: Notices include shave gel, shave foam, and volumizing foam
3 Notices
Nitrous Oxide
Consumer Products
Product Category
Notice(s)
Alleged Chemicals
Plastic Pouches, Bags, and Accessories: Notices include children’s bags, beauty bags, bento bags, fanny packs, backpacks, wallets, picking bags, weight stabilizing bags, travel bags, rescuer guide packs, shoe covers, and cases for wheel sets
26 Notices
Di(2-ethylhexyl)phthalate (DEHP), Diisononyl phthalate (DINP), and Di-n-butyl phthalate (DBP)
Miscellaneous Consumer Products: Notices include orthodontic kits, keychains, back scratchers, safety flags, vinyl banners, engraved wax sealers, steering wheel covers, lamps, stethoscopes, salt and pepper shakers with PVC components, luggage tag, and vinyl roll holders
26 Notices
Di(2-ethylhexyl)phthalate (DEHP), Diisononyl phthalate (DINP), Di-n-butyl phthalate (DBP), and Lead
Hardware and Home Improvement Products: Notices include long handle hooks, garden hose splitters, coatings and paints, soldering wire, tools with PVC grips, pressure gauge, thermocouples, wing nuts, pop-up drains, propane tank adapter, and thread tape
23 Notices
Lead and Lead Compounds, Di(2-ethylhexyl)phthalate (DEHP), Diisononyl phthalate (DINP), and Perfluorooctanoic Acid (PFOA)
Clothing and Shoes: Notices include gloves made with leather, bucket hats, sandals with PVC components, golf gloves, weatherproof jackets, slides, fuzzy socks, and ski pants
22 Notices
Di(2-ethylhexyl)phthalate (DEHP), Chromium (hexavalent compounds), Perfluorooctanoic Acid (PFOA),
and Bisphenol A (BPA)
Glassware, Metals, and Ceramics: Notices include mugs, glass sets, blue multi-colored glass, metal and glass organizers, spoon rests, shakers, and soap dispenser/sponge holders
19 Notices
Lead and Lead Compounds
Miscellaneous Consumer Products: Notices include shower curtains, tablecloths, pillows, pet beds, athletic bandages, and outdoor cushions
10 Notices
Perfluorooctanoic Acid (PFOA)
Hobby Items: Notices include artist paste paints, art panels, lens mounts, pickleball paddles, jump rope, molding cream, and golf storage boot
8 Notices
Di(2-ethylhexyl)phthalate (DEHP), Di-n-butyl phthalate (DBP), Lead, Diethanolamine, and Perfluorooctanoic Acid (PFOA)
Coal Tar Epoxy
1 Notice
Bisphenol A (BPA), Epichlorohydrin, Ethylbenzene, soots, tar and mineral oils (coal tar)
There are numerous defenses to Prop. 65 claims, and proactive measures that industry can take prior to receiving a Prop. 65 Notice in the first place. Keller and Heckman attorneys have extensive experience in defense of Prop. 65 claims and in all aspects of Prop. 65 compliance and risk management. We provide tailored Proposition 65 services to a wide range of industries, including food and beverage, personal care, consumer products, chemical products, e-vapor and tobacco products, household products, plastics and rubber, and retail distribution.
New Artificial Intelligence (AI) Regulations and Potential Fiduciary Implications
Fiduciaries should be aware of recent developments involving AI, including emerging and recent state law changes, increased state and federal government interest in regulating AI, and the role of AI in ERISA litigation. While much focus has been on AI’s impact on retirement plans, which we previously discussed here, plan fiduciaries of all types, including health and welfare benefit plans, must also stay informed about recent AI developments.
Recent State Law Changes
Numerous states recently codified new laws focusing on AI, some of which regulate employers’ human resource decision-making processes. Key examples include:
California – In 2024, California enacted over 10 AI-related laws, addressing topics such as:
The use of AI with datasets containing names, addresses, or biometric data;
How one communicates health care information to patients using AI; and
AI-driven decision-making in medical treatments and prior authorizations.
For additional information on California’s new AI laws, see Foley’s Client Alert, Decoding California’s Recent Flurry of AI Laws.
Illinois – Illinois passed legislation prohibiting employers from using AI in employment activities in ways that lead to discriminatory effects, regardless of intent. Under the law, employers are required to provide notice to employees and applicants if they are going to use AI for any workplace-related purpose.
For additional information on Illinois’ new AI law, see Foley’s Client Alert, Illinois Enacts Legislation to Protect against Discriminatory Implications of AI in Employment Activities.
Colorado – The Colorado Artificial Intelligence Act (CAIA), effective February 1, 2026, mandates “reasonable care” when employers use AI for certain applications.
For additional information on Colorado’s new AI law, see Foley’s Client Alert, Regulating Artificial Intelligence in Employment Decision-Making: What’s on the Horizon for 2025.
While these laws do not specifically target employee benefit plans, they reflect a trend toward states regulating human resource practices broadly, are aimed at regulating human resource decision-making processes, and are part of an evolving regulatory environment. Hundreds of additional state bills were proposed in 2024, along with AI-related executive orders, signaling more forthcoming regulation in 2025. Questions remain about how these laws intersect with employee benefit plans and whether federal ERISA preemption could apply to state attempts at regulation.
Recent Federal Government Actions
The federal government recently issued guidance aimed at preventing discrimination in the delivery of certain healthcare services and completed a request for information (RFI) for potential AI regulations involving the financial services industry.
U.S. Department of Health and Human Services (HHS) Civil Rights AI Nondiscrimination Guidance – HHS, through its Office for Civil Rights (OCR), recently issued a “Dear Colleague” letter titled Ensuring Nondiscrimination Through the Use of Artificial Intelligence and Other Emerging Technologies. This guidance emphasizes the importance of ensuring that the use of AI and other decision-support tools in healthcare complies with federal nondiscrimination laws, particularly under Section 1557 of the Affordable Care Act (Section 1557).
Section 1557 prohibits discrimination on the basis of race, color, national origin, sex, age, or disability in health programs and activities receiving federal financial assistance. OCR’s guidance underscores that healthcare providers, health plans, and other covered entities cannot use AI tools in a way that results in discriminatory impacts on patients. This includes decisions related to diagnosis, treatment, and resource allocation. Employers and plan sponsors should note that this guidance applies to a subset of health plans, including those that fall under Section 1557, but not to all employer-sponsored health plans.
Treasury Issues RFI for AI Regulation – In 2024, the U.S. Department of Treasury published an RFI on the Uses, Opportunities, and Risks of Artificial Intelligence in the Financial Services Sector. The RFI included several key considerations, including addressing AI bias and discrimination, consumer protection and data privacy, and risks to third-party users of AI. While the RFI has not yet led to concrete regulations, it underscores federal attention to AI’s impact on financial and employee benefit services. The ERISA Industry Committee, a nonprofit association representing large U.S. employers in their capacity as employee benefit plan sponsors, commented that AI is already being used for retirement readiness applications, chatbots, portfolio management, trade executions, and wellness programs. Future regulations may target these and related areas.
AI-Powered ERISA Litigation
Potential ERISA claims against plan sponsors and fiduciaries are being identified using AI. In just one example, an AI platform, Darrow AI, claims to be:
“designed to simplify the analysis of large volumes of data from plan documents, regulatory filings, and court cases. Our technology pinpoints discrepancies, breaches of fiduciary duty, and other ERISA violations with accuracy. Utilizing our advanced analytics allows you to quickly identify potential claims, assess their financial impact, and build robust cases… you can effectively advocate for employees seeking justice regarding their retirement and health benefits.”
Further, this AI platform claims it can find violations affecting many types of employers, whether a small business or a large corporation, by analyzing diverse data sources, including news, SEC filings, social networks, academic papers, and other third-party sources.
Notably, health and welfare benefit plans are also emerging as areas of focus for AI-powered ERISA litigation. AI tools are used to analyze claims data, provider networks, and administrative decisions, potentially identifying discriminatory practices or inconsistencies in benefit determinations. For example, AI could highlight patterns of bias in prior authorizations or discrepancies in how mental health parity laws are applied.
The increasing sophistication of these tools raises the stakes for fiduciaries, as they must now consider the possibility that potential claimants will use AI to scrutinize their decisions and plan operations with unprecedented precision.
Next Steps for Fiduciaries
To navigate this evolving landscape, fiduciaries should take proactive steps to manage AI-related risks while leveraging the benefits of these technologies:
Evaluate AI Tools: Undertake a formal evaluation of artificial intelligence tools utilized for plan administration, participant engagement, and compliance. This assessment includes an examination of the algorithms, data sources, and decision-making processes involved, including an assessment to ensure their products have been evaluated for compliance with nondiscrimination standards and do not inadvertently produce biased outcomes.
Audit Service Providers: Conduct comprehensive audits of plan service providers to evaluate their use of AI. Request detailed disclosures regarding the AI systems in operation, focusing on how they mitigate bias, ensure data security, and comply with applicable regulations.
Review and Update Policies: Formulate or revise internal policies and governance frameworks to monitor the utilization of AI in operational planning and compliance with nondiscrimination laws. These policies should outline guidelines pertaining to the adoption, monitoring, and compliance of AI technologies, thereby ensuring alignment with fiduciary responsibilities.
Enhance Risk Mitigation:
Fiduciary Liability Insurance: Consider obtaining or enhancing fiduciary liability insurance to address potential claims arising from the use of AI.
Data Privacy and Security: Enhance data privacy and security measures to safeguard sensitive participant information processed by AI tools.
Bias Mitigation: Establish procedures to regularly test and validate AI tools for bias, ensuring compliance with anti-discrimination laws.
Integrate AI Considerations into Requests for Proposals (RFPs): When selecting vendors, include specific AI-related criteria in RFPs. This may require vendors to demonstrate or certify compliance with state and federal regulations and adhere to industry best practices for AI usage.
Monitor Legal and Regulatory Developments: Stay informed about new state and federal AI regulations, along with the developing case law related to AI and ERISA litigation. Establish a process for routine legal reviews to assess how these developments impact plan operations.
Provide Training: Educate fiduciaries, administrators, and relevant staff on the potential risks and benefits of AI in plan administration, emerging technologies and the importance of compliance with applicable laws. The training should provide an overview of legal obligations, best practices for implementing AI, and strategies for mitigating risks.
Document Due Diligence: Maintain comprehensive documentation of all steps to assess and track AI tools. This includes records of audits, vendor communications, and updates to internal policies. Clear documentation can act as a crucial defense in the event of litigation.
Assess Applicability of Section 1557 to Your Plan: Health and welfare plan fiduciaries should determine whether your organization’s health plan is subject to Section 1557 and whether OCR’s guidance directly applies to your operations, and if not, confirm and document why not.
Fiduciaries must remain vigilant regarding AI’s increasing role in employee benefit plans, particularly amid regulatory uncertainty. Taking proactive measures and adopting robust risk management strategies can help mitigate risks and ensure compliance with current and anticipated legal standards. By dedicating themselves to diligence and transparency, fiduciaries can leverage the benefits of AI while safeguarding the interests of plan participants. At Foley & Lardner LLP, we have experts in AI, retirement planning, cybersecurity, labor and employment, finance, fintech, regulatory matters, healthcare, and ERISA. They regularly advise fiduciaries on potential risks and liabilities related to these and other AI-related issues.
FDA Announces Red No. 3 Authorizations to be Revoked as Matter of Law, not Safety
Today FDA announced that it is revoking the color additive authorizations for Red No. 3 in food (including dietary supplements) and ingested drugs based on evidence showing that Red No. 3 is carcinogenic to male rats (not humans, or even female rats) and the so-called “Delaney Clause” of the Federal Food, Drug, and Cosmetic Act (FD&C Act) which prevents the agency from authorizing an additive that has been found to cause cancer in humans or animals. The Delaney Clause as it pertains to color additives can be found in section 721(b)(5)(B) of the FD&C Act (21 USC 379e(b)(5)(B)) and a similar provision pertaining to food additives can be found in section 409(c)(3)(A) (21 USC 348(c)(3)(A)).
FDA’s announcement makes clear that the currently available scientific information does not support safety concerns regarding the use of Red No. 3 and that its decision was one it feels it was required to make based on the extremely broad scope of the Delaney Clause, which was added to the FD&C Act over 60 years ago and has not been updated since to keep up with new scientific understandings of cancer.
More specifically, consistent with its prior statements on Red No. 3, FDA concluded that Red No. 3 causes cancer in male rats at high doses by increasing the levels of a thyroid hormone (TSH). However, this mechanism of action is not relevant to humans; rats are much more sensitive to changes in TSH levels and studies in humans have not demonstrated that Red No. 3 changes thyroid hormone levels, including TSH. Finally, carcinogenicity of Red No. 3 has not been observed when female rats were tested, or when either sex of mice, gerbils, or dogs were tested.
The decision will be published in the federal register tomorrow (01/16/2025), but a pre-publication version of the federal register notice is available here. Manufacturers using Red No. 3 in food will have until January 15, 2027 to reformulate their products while manufacturers using Red No. 3 in ingested drugs will have until January 18, 2028 to reformulate.
This follows California’s ban of Red No. 3 with the signing of the California Food Safety Act in 2023 by Gov. Gavin Newsom which will go into effect in 2027 as well.
The Telehealth Extension Has Ended…For Now
During the COVID-19 crisis, newly-created relief allowed first dollar coverage for telehealth services under a high deductible health plan (HDHP) without ruining health savings account (HSA) eligibility. That relief was extended for plan years beginning prior to January 1, 2025. You can read our articles regarding the initial relief and subsequent extensions here, here, and here.
An earlier version of the 2025 budget bill included a two-year extension of this HSA telehealth safe harbor relief. However, that provision did not make it into the slimmed down version of the budget bill that was signed by President Biden in late December. The slimmed down budget bill was intended to serve as a stop gap to keep the Federal government running through March 14, 2025. Industry members are hopeful that when budget talks resume, a telehealth extension will be a part of that discussion.
For now, the telehealth relief has ended. For plan years beginning on or after January 1, 2025, pre-HDHP deductible coverage for telehealth services will disqualify an individual from contributing to an HSA unless another exception applies.
New York’s Reproductive Health Handbook Notice Requirement Reinstated
Don’t finalize your 2025 handbooks just yet!
On January 2, 2025, the United States Court of Appeals for the Second Circuit vacated a permanent injunction, which had blocked a requirement that New York employers with employee handbooks include a notice against discrimination based on reproductive health care choices. As a result, handbooks covering New York employees must again include such notices.
The notice requirement originates from a series of legislation intended to protect reproductive health rights enacted on November 8, 2019. As we previously reported, one of the bills (A584/S660) added Section 203-e to the New York labor law, which prohibits employers from discriminating against employees based on an employee’s or their dependents’ sexual and reproductive health choices, including their choice to use or access a particular drug, device, or medical service. The law also prohibits employers from accessing such information without prior consent, and directed New York employers with employee handbooks to include a notice of employee rights and remedies. Although the law took effect immediately upon passage, a second bill (S4413) delayed the effective date of the notice requirement until January 2020.
A little more than two years later, the U.S. District Court for the Northern District of New York blocked the notice requirement. In CompassCare et al. v. Cuomo, several faith-based employers challenged Section 203-e in its entirety as violative of the First Amendment to the United States Constitution. Although the District Court dismissed most of the claims, on March 29, 2022, the court permanently enjoined enforcement of the notice requirement stating that it “would compel [the plaintiffs] to promote a message about conduct contrary to their religious perspectives” as they relate to reproductive health choices, such as birth control and abortion. The court found that, while New York has a compelling interest in protecting employee privacy, the State had not demonstrated that the notice requirement was the least restrictive means of achieving that interest. For example, employers could inform employees of their rights and the remedies under the law in other ways, such as placing posters at the job site, or advertising the statutory provision generally.
On appeal nearly three years later, the Second Circuit vacated the permanent injunction, thus reinstating the handbook notice requirement. The Second Circuit panel found that the requirement is similar to other state and federal laws requiring workplace disclosures and noted that while the policy judgments motivating Section 203-e may be “controversial”, so are those underlying Title VII or minimum wage laws, but that does not make an employer’s obligation to comply controversial. The Second Circuit also stated that the notice requirement does not prevent employers from otherwise communicating to employees, in their handbooks or elsewhere, their political or religious views, including their disagreement with Section 203-e.
In light of the Second Circuit’s decision, New York employers should review and revise their employee handbook to include a notice of employees’ reproductive health rights and remedies as provided by Section 203-e. The law does not provide specific language to include – and New York has not published a model notice or any further guidance on the law to date – thus, employers should consult employment counsel to ensure that their handbook notice satisfies the law’s requirements.
2024 Hatch-Waxman Year in Review
Introduction
In 2024, the Hatch-Waxman Act continued to play a critical role in the U.S. pharmaceutical landscape, driving the dynamics between brand-name drugmakers and generics. This landmark legislation, enacted to encourage innovation while ensuring access to affordable medications, remained a focal point for numerous legal battles and regulatory shifts. Key decisions throughout the year have refined interpretations of its provisions, influencing patent challenges, market exclusivities, and the pathway for generics. As the pharmaceutical sector navigates evolving market pressures, agency action, and possible legislation, the legal contours of the Hatch-Waxman Act continued to impact both the business and legal strategies of pharmaceutical companies in 2024.
The Year By Numbers
In the year 2024, 312 complaints were filed initiating Hatch-Waxman litigation (compared to 259 in 2023)1:
As evident above, the overwhelming majority of ANDA complaints were filed in the District of Delaware and the District of New Jersey. This common trend remains consistent for the same reasons these district courts have always been hubs for ANDA litigation: most pharmaceutical companies are incorporated in Delaware and are commonly headquartered in New Jersey. Furthermore, because these two jurisdictions handle the majority of ANDA litigation, the local patent rules and proclivities of judges within these districts generally account for the unique procedural complexities that large-scale Hatch-Waxman litigation can impose on these dockets.
Given that Hatch-Waxman litigation is statutorily decided at the bench if it goes to trial, it behooves all litigants to have matters handled by judges experienced in the technical subject matter. As shown above, almost 50% of all ANDA complaints filed were assigned to one of five judges, ensuring that those judges have familiarity with common Hatch-Waxman substantive and procedural issues, and usually leading to a rapport between those judges and the attorneys that frequently litigate in front of them.
In 2024, 283 on-going Hatch-Waxman litigations were either resolved or terminated.2 There was a slight decrease in settlements in 2024: 39% of terminated matters in 2024 compared to 50% in 2023.3 Innovator companies (i.e., NDA & patent holders) were considered to have prevailed on issues 20% of the time, whereas generic companies were considered to have prevailed on issues only 2% of the time (i.e., those decisions excluding settlements and procedural resolutions). While these statistics may suggest that innovator companies find favorable resolutions more frequently than generic manufacturers, generics generally may be more inclined to seek settlement when perceiving a likely favorable outcome, rather than continue litigation. This trend existed in 2023 and remained in 2024.
Looking at patent findings from 2024 (below4), evidently very few ANDA cases were decided at summary judgment in 2024, a frequency from which few conclusions can be drawn. When cases went to trial, however, we saw a finding of infringement more frequently than noninfringement, and validity was upheld more frequently than not. Of those that were held invalid at trial, most were decided on obviousness grounds. Granted, however, these numbers don’t consider invalidity positions that were dropped due to case narrowing prior to trial, rather than on the merits.
This contrasts slightly to the results from 2023 (below5):
Namely, judges were seemingly more reticent to find patents invalid at summary judgment in 2024, while they did so three times in 2023 – again, however, a small sample size. In good news for innovator companies, district courts not only held patents invalid at trial far less frequently in 2024 compared to the year prior: 4 of 17 (24%) and 9 of 15 (60%), respectively. District courts also found infringement of valid patents at trial slightly more in 2024 compared to the year prior: 9 of 13 (69%) versus 6 of 10 (60%), respectively.
Federal Circuit Decisions and the Greater Context In Which They Fit
We saw a slight uptick in Hatch-Waxman decisions from the Federal Circuit last year (7 in 2024 compared to 5 in both 2023 and 2022), some of which significantly affect going forward how practitioners and in-house counsel manage and plan their IP strategies, expand their portfolios through prosecution, and preserve existing exclusivities in the federal courts and in front of the Patent Trial and Appeal Board. Some of the decisions we’ve seen from the Federal Circuit in 2024 were also germane to broader agency and legislative proposals that could come to fruition in 2025, as discussed below.
Edwards Lifesciences v. Meril Life Sciences6& the Safe Harbor Provision
Holding: The Hatch-Waxman safe harbor applied to the importation of two demonstration samples to a medical conference for the purpose of recruiting clinical investigators to support FDA approval.
Although not a decision surrounding the filing of an ANDA, the Federal Circuit began their 2024 Hatch-Waxman jurisprudence addressing the safe harbor provision, 35 U.S.C. § 271(e)(1): a valuable mechanism for fostering innovation in the pharmaceutical space. Federal Circuit precedent has interpreted the provision as broad, applying “as long as there is a reasonable basis for believing that the use of the patented invention will produce the types of information that are relevant to an FDA submission,”7 and even extending to activities which may be promotional rather than regulatory, but “where those activities are consistent with the collection of data necessary for filing an application with the FDA.”8 Here, Judges Stoll, Cunningham, and Lourie (dissenting) addressed whether the importation of two demonstration-only transcatheter heart valves for a conference during the process of pre-market approval was protected by the safe harbor, and ultimately affirmed precedent.9 As Judge Stoll put it, the question is not why or how the devices were imported or used, but whether the importation was for a use reasonably related to submitting information to the FDA.10 It was here. On appeal from a grant of summary judgment of no infringement, the Federal Circuit affirmed that there was no genuine dispute of material fact that Meril imported the devices for purposes reasonably related to recruiting investigators during pre-market approval processes and thus was covered by the safe harbor provision.11 For innovator companies, especially those in crowded commercial spaces where the risk of “brand-to-brand” litigation is higher, the safe harbor’s broad applicability to a variety of pre-approval activities under the ”reasonably related” standard offers peace of mind throughout early stages of product development; however, practitioners should advise their clients that the safe harbor is less helpful post-FDA approval, where routine submissions aren’t generally afforded the same protection.12
While courts may view the Hatch-Waxman safe harbor as offering a “wide berth,”13 U.S. patent law generally has a particularly narrow experimental use defense to patent infringement.14 The Edwards decision was followed months later by a request for public commentary by the United States Patent and Trademark Office (USPTO) on the potential legislative codification of the experimental use exception.15 To date, statutory experimental use defenses are confined in the U.S. to the Hatch-Waxman Act16 and the Plant Variety Protection Act,17 but are codified in a much broader fashion in other leading IP countries, such as Germany, China, and India. Feedback to the USPTO’s request was mixed; proponents of further codification suggested the exception was overly narrow, vague, or detrimental to US innovation on the global scale, whereas those with opposing viewpoints generally suggested the status quo was sufficient. At this time, the USPTO has not taken any further public action on the topic, but don’t be surprised if we see legislation promoting American innovation in 2025, such as an expanded codification of the experimental use defense.
Salix Pharmaceuticals v. Norwich Pharmaceuticals,18Post-trial Section VIII Carve-outs, and the Obviousness of Polymorph Patents
Holding: The district court did not err in denying the generic’s motion to modify judgment after amending its ANDA to remove an infringing indication after trial; the district court also did not err in finding that a person of ordinary skill in the art would have a reasonable expectation of success obtaining certain polymorph forms of rifaximin.
One month later, the first ANDA decision came from the Federal Circuit from Judges Lourie, Chen, and Cunningham (dissenting in part), who issued a surprising decision in light of (but not contradictory to) previous rulings on polymorph patents, while also addressing a unique post-trial tactic by the ANDA filer to gain earlier entry into the market. With respect to the former, Federal Circuit precedent has made it clear that finding polymorph claims obvious is a tall task given the unpredictability of chemical polymorphism and therefore the lack of reasonable expectation of success, as discussed in Grunenthal GMBH v. Alkem19 (2019) and Pharmacyclics v. Alvogen20 (2022). However, unique to this case were the “distinct factual predicates” that justified the district court’s obviousness finding.21 The prior art here contained examples which disclosed in detail the process that would produce the claimed polymorph, turn demonstrating a reasonable expectation of success in doing so.22 Therefore, unlike in previous § 103 decision on polymorphs, those at issue here was appropriately found to be obvious. Separately, Norwich’s ANDA sought to market generic Xifaxan for three indications: travelers’ disease, hepatic encephalopathy (HE) and irritable-bowel syndrome with diarrhea (IBS-D).23 However, when the district court ordered that the ANDA would not be approved until the expiry of the HE patents (which were found infringed), Norwich amended its ANDA post-trial to remove the infringing HE indication and sought to modify the judgment and gain earlier market entry.24 Both the district court and Federal Circuit rejected this attempt.25 The latter held that “it [was] not the potential use that of the drug for HE that is the relevant infringement,” but instead “the submission of the ANDA that included an infringing use,” and therefore “[t]hat the ANDA further recited a non-patent-protected indication does not negate the infringement resulting from the ANDA’s submission.”26 Further, allowing amendment of an ANDA at the Rule 60 stage is in the discretion of the district court, and the Federal Circuit’s affirmation of the district court’s decision created strong precedent that determining “whether an ANDA applicant has successfully carved out language from a label to turn infringement into non-infringement” “would essentially be a second litigation,” and is “inequitable and inappropriate.”27
This decision offers two key takeaways for counsel for both innovators and generics: for the former, the nonobviousness of polymorph patents is not a guaranteed, despite the unique, unpredictable nature of the science and the general position of related jurisprudence. While finding polymorph patents obvious is still a significant challenge given their general nature, it is possible for the right facts to line up correctly in a § 103 analysis. For generic companies, future tactical attempts to carve out infringing indications post-trial now must overcome cut-and-dry precedent suggesting the futility of the practice to gain earlier market entry.
Amarin Pharma v. Hikma Pharmaceuticals28 & Skinny Labels
Holding: The complaint plausibly pleaded induced infringement based on the label and public statements made by the generic manufacturer.
The next panel from the Federal Circuit (Moore, Lourie, Albright) next dealt with what seemed like a section viii carve out ANDA case, but was rather a “run-of-the-mill induced infringement case.”29 The generic product, an icosapent ethyl already on the market, was approved for only one of the two indications (treatment of severe hypertriglyceridemia) that the NDA product (Vascepa) had been approved for, but included no limitation of use as to the second indication, and the generic manufacturer had made repeated public statements referring to itself as the “generic Vascepa,” despite being approved for only half the indications.30 Unique to this case was that it was appealed from the motion to dismiss stage, and thus discovery had not occurred.31 Not in dispute however was that the complaint sufficiently alleged direct infringement, knowledge, and intent, and thus the Federal Circuit’s decision focused on whether an “inducing act” was sufficiently alleged – it was.32 Reversing the district court’s dismissal, the Federal Circuit managed to walk along the “careful balance struck by the Hatch-Waxman Act regarding section viii carve-outs,” emphasizing that this decision did not “effectively eviscerate section viii-carveouts,” as argued by Hikma, and was instead “limited to the allegations” and “guided by the standard of review appropriate for this stage of the proceedings.”33 Given those explicitly limiting statements, this decision does little to affect true section viii jurisprudence under the Hatch-Waxman Act, and thus for practitioners, reliance on cases such as GlaxoSmithKline v. Teva (2021)34 is still appropriate for skinny label analyses.
Following the Salix and Amarin decisions in 2024 we saw new related agency action from the FDA and year-end legislation. In July 2024, the FDA rejected a citizen’s petition from Novartis requesting the FDA reject ANDAs for generic Entresto, instead allowing generic manufacturers to add new language to their label, not included in the currently approved indication, that would effectively narrow the subset of patients for which use of the generic product is appropriate.35 In this case, inclusion of the language “patients with…reduced ejection fraction” was permissible as it therefore excluded “patients with…preserved ejection fraction,” which is patent protected.36 This decision was affirmed by the District Court for the District of Columbia.37 To wrap up 2024, we also saw the introduction of a bill titled the “Skinny Labels, Big Savings Act” on December 17, which seeks to provide safe harbor protection to generics and biosimilars using skinny labels in certain contexts.38
Allergan USA v. MSN Laboratories39 & Obviousness-type Double Patenting
Holding: First-filed, first-issued, later-expiring patent claims were not invalid for obviousness-type double patenting over later-filed, later-issued, earlier-expiring reference claims.
In August, the Federal Circuit clarified its 2023 In re Cellect decision40 which, at the time, served to massively upheave the doctrine of obviousness-type double patenting (ODP), patent term adjustments (PTA), and terminal disclaimers. However, Judges Lourie, Dyk, and Reyna reeled the impacts of that decision back in. Although the district court considered itself “bound” by the In re Cellect holding, the Federal Circuit distinguished the two as addressing different questions.41 Here, the question was “can a first-filed, first-issued, later-expiring claim be invalidated by a later-filed, later-issued, earlier-expiring reference claim having a common priority date,” to which the Court decided “no.”42 The Cellect decision, however, was boiled down to establishing the rule that “when it comes to evaluating ODP on a patent that has received PTA, the relevant expiration date is the expiration date including PTA—not the original expiration date measured twenty years from the priority date.”43 Practitioners now know that Cellect does not require a patent to be invalidated by reference patents simply because it expires later. The doctrine of obviousness-type double patent serves to prohibit the extension of a first patent by subsequently filed patently indistinct patents; it does not serve to cut short first-filed patents with duly received PTA, simply because later-filed patents expire first.
This decision also follows a May proposal from the USPTO to implement a new rule on terminal disclaimers, such that a terminal disclaimer would include a provision aiming to reduce the costs associated with challenging patent families under ODP.44 The rule proposed that when filing a terminal disclaimer, a patentee must agree that a patent subject to a terminal disclaimer would only be enforceable if it was not tied through such a disclaimer to a patent which had otherwise been held unpatentable or invalid.45 The rule avoids the issue of having to invalidate multiple related patents separately, and if implemented, may significantly impact how practitioners approach continuation patents and handle large patent families within a portfolio. We may see a decrease in continuation applications, and instead see applications claiming much broader scopes and an increase in divisional applications.
Astellas Pharma v. Sandoz46 & Patent Eligibility
Holding: Courts may not sua sponte consider patent eligibility as grounds for patent invalidity.
In September, the Federal Circuit made clear that issues of patent eligibility under Section 101 cannot be decided sua sponte by district courts. Known as the principle of party presentation, there are circumstances in which a court may take “a modest initiating role” in shaping litigation,47 but addressing patent eligibility when not raised by a party is not one such circumstance. Here, patent eligibility was never raised during the course of the litigation, but the court considered it anyway in its final decision.48 While the district court phrased its decision in such a manner that parties may not “consent around the bounds of patent eligibility,” the Federal Circuit Judges Lourie, Prost, and Reyna made clear that patent eligibility is not a threshold issue akin to subject-matter jurisdiction, but instead is entitled to the presumption of validity, as is the case with other grounds of validity.49 While perhaps this decision offers greater direction to courts than counsel, it serves as a polite reminder to practitioners that any invalidity defense not raised is waived.
The topic of patent eligibility was particularly ripe in 2024, and continuing into 2025, especially with the growth of artificial intelligence (AI). Although not a topic that is overly adjacent to Hatch-Waxman litigation, counsel for both innovator and generic companies should remain cognizant of updated guidance from the USPTO, such as that which issued in July.50 A full summary of the guidance can be found here. Generic companies do appear to be raising § 101 invalidity grounds less frequently in recent years; however, counsel should nonetheless stay informed on both procedural and substantive developments in patent eligibility jurisprudence.
Galderma Laboratories v. Lupin51 & Bioequivalence Data and In Vitro Testing
Holding: The district court did not err in holding that the plaintiff had not proven infringement by relying on its in vitro testing and bioequivalence.
As one of two December ANDA decisions in 2024, the Federal Circuit analyzed whether in vitro testing and bioequivalence were sufficient to establish literal infringement or infringement under the doctrine of equivalence. The result? They aren’t. Although likely not a hard-and-fast rule that in vivo and in vitro results are not comparable, the Federal Circuit found no clear error in the district court’s conclusion as such in this particular case, finding that Galderma improperly drew conclusions about in vivo behavior from in vitro testing.52 Evidently, the issue was a failure of proof, rather than scientific incomparability.53 Further, unique to the facts of this case, although with a slightly broader applicability generally, under a doctrine of equivalents analysis, a showing of bioequivalence, at most, shows substantially the same result, but fails to show substantially the same function or substantially the same way, as is required under the “function, way, result” test.54 This decision highlights the importance of accurate and reliable testing to prove infringement, as well as fulsome expert testimony relaying as such.
Teva v. Amneal Pharmaceuticals55 & Orange-Book Listings
Holding: Patents directed towards inhaler devices were improperly listed in the Orange Book.
Wrapping up 2024, the Federal Circuit addressed a key issue pressing innovator pharmaceutical companies: the propriety of Orange Book listings. In this case, Teva had listed patents directed to inhaler devices in the Orange Book in order to delay the entry of generic products to the market.56 Because such patents “contain no claim for the active ingredient at issue,” Judges Prost, Taranto, and Hughes affirmed the district court’s delisting order.57 In what ultimately came down to issues of statutory interpretation, the Federal Circuit rejected arguments that a patent is properly listed if it “reads on” the approved drug or claims any component of a drug.58 Practitioners now know this is not the case. First, “the fact that an NDA could infringe a patent does not mean that the patent ‘claims’ the underlying drug within the meaning of the listing provision.”59 And second, “[t]o list a patent in the Orange Book, that patent must, among other things, claim the drug for which the applicant submitted the application and for which the application was approved,” i.e., the active ingredient.60 While NDA holders are keen to protect their products from generic entry into the market, this decision from the Federal Circuit affirms that there is a limit to the Orange Book, and NDA holders would be wise to ensure any such listings do in fact claim the active ingredient at issue.
This decision comes in the wake of threats by the Federal Trade Commission and new de-listing policies. For example, in September 2023, the FTC issued a new policy61 stating that improper Orange Book listings may constitute a violation of Section 5 of the FTC Act, and in November 2023, the FTC announced a plan62 to challenge over 100 Orange Book Listings and a further 300 listings in April 202463. Many companies have received warning letters from the FTC, only some of whom have voluntarily delisted at-risk Orange Book patents however. The financial detriment of doing so is clear, and other companies have therefore pushed back, arguing compliance with the listing provisions. The FTC appears to be predominantly targeting medical device patents, such as those in Teva v. Amneal, but for the most part, action by the FTC remains limited to issuing policies and sending letters to NDA holders. Further, it has yet to be determined whether this is an appropriate exercise of agency power, especially in the wake of the Supreme Court’s goodbye to Chevron deference.64
To conclude, many of the Federal Circuit’s Hatch-Waxman decisions in 2024 reshaped how pharmaceutical companies and their counsel address patent prosecution, litigation, and portfolio management, especially in view of the broader regulatory, legislative, agency-based changes that may occur in 2025 and beyond. As patent law continues to evolve, these cases will serve as critical touchstones in understanding the future of pharmaceutical patents and the broader implications for drug pricing and accessibility in the years to come.
1 LexMachina stats showing 312 federal district court cases with “Patent:ANDA” case tag, filed between ”2024-01-01 and 2024-12-31″) (Compare with LexMachina stats showing 259 federal district court cases with ”Patent:ANDA” case tag, filed between ”2023-01-01 and 2023-12-31″.2 LexMachina stats showing 283 federal district court cases with “Patent:ANDA” case tag, terminated between ”2024-01-01 and 2024-12-31″.3 Compare supra with LexMachina stats showing 284 federal district court cases with “Patent:ANDA” case tag, terminated between ”2023-01-01 and 2023-12-31″.4 LexMachina stats showing 91 findings in cases with “Patent:ANDA” case tag, with ”Infringement, Invalidity, No Infringement, No Invalidity, No Unenforceability, or Unenforceability” as patent findings, with findings decided between ”2024-01-01 and 2024-12-31″.5 LexMachina stats showing 91 findings in cases with “Patent:ANDA” case tag, with ”Infringement, Invalidity, No Infringement, No Invalidity, No Unenforceability, or Unenforceability” as patent findings, with findings decided between ”2023-01-01 and 2023-12-31″.6 Edwards Lifesciences Corp. v. Meril Life Scis. Pvt. Ltd., 96 F.4th 1347 (Fed. Cir. 2024).7 Amgen Inc. v. Hospira, Inc., 944 F.3d 1327, 1338 (Fed. Cir. 2019).8 Momenta Pharm., Inc. v. Teva Pharm. USA Inc., 809 F.3d 610, 619 (Fed. Cir. 2015).9 Supra, note 6, at 1351.10 Id. at 135311 Id. at 1355.12 Classen Immunotherapies v. Biogen IDEC, 659 F.3d 1057, 1070 (Fed. Cir. 2011).13 Merck KGaA v. Integra Lifesciences I, Ltd., 545 U.S. 193, 202 (2005).14 See, e.g., Madey v. Duke Univ., 307 F.3d 1351 (Fed. Cir. 2002).15 89 Fed. Reg. 53963.16 35 U.S.C. § 271(e)(1).17 7 U.S.C. § 2544.18 Salix Pharms., Ltd. v. Norwich Pharms. Inc., 98 F.4th 1056 (Fed. Cir. 2024).19 Grunenthal GMBH v. Alkem Lab’ys Ltd., 919 F.3d 1333 (Fed. Cir. 2019).20 Pharmacyclics LLC v. Alvogen, Inc., No. 2021-2270, 2022 WL 16943006 (Fed. Cir. Nov. 15, 2022).21 Supra, note 18, at 1065.22 Id. at 1066-67.23 Id. at 1060.24 Id. at 1068-69.25 Id. at 1069.26 Id. at 1068.27 Id. at 1069.28 Amarin Pharma, Inc. v. Hikma Pharms. USA Inc., 104 F.4th 1370 (Fed. Cir. 2024).29 Id. at 1377.30 Id. at 1372-74.31 Id. at 1377.32 Id. at 1378.33 Id. at 1381.34 GlaxoSmithKline LLC v. Teva Pharms. USA, Inc., 7 F.4th 1320 (Fed. Cir. 2021).35 Final Response Letter from FDA CDER to Novartis Pharmaceuticals Corporation, Docket FDA-2022-P02228 (24 Jul. 2024), https://downloads.regulations.gov/FDA-2022-P-2228-0015/attachment_1.pdf. 36 See id.37 Novartis Pharms. Corp. v. Becerra, No. 24-CV-02234 (DLF), 2024 WL 4492072 (D.D.C. Oct. 15, 2024).38 Sens. Hickenlooper, Welch, Cotton, & Collins, Skinny Labels, Big Savings Act, https://www.hickenlooper.senate.gov/wp-content/uploads/2024/12/Skinny-Labels.pdf.39 Allergan USA, Inc. v. MSN Lab’ys Priv. Ltd., 111 F.4th 1358 (Fed. Cir. 2024).40 In re: Cellect, LLC, 81 F.4th 1216 (Fed. Cir. 2023).41 Supra, note 39, at 1368.42 Id. at 1366.43 Id. at 1369.44 89 Fed.Reg. 4043945 Id.46 Astellas Pharma, Inc. v. Sandoz Inc., 117 F.4th 1371 (Fed. Cir. 2024).47 United States v. Sineneng-Smith, 590 U.S. 371, 376 (2020).48 Supra, note 46, at 1376.49 Id. at 1378.50 89 Fed.Reg. 5812851 Galderma Lab’ys, L.P. v. Lupin Inc., 122 F.4th 902 (Fed. Cir. 2024).52 Id. at 908.53 Id. at 908.54 Id. at 910.55 Teva Branded Pharm. Prods. R&D, Inc. v. Amneal Pharms. of New York, LLC, No. 2024-1936, 2024 WL 5176737 (Fed. Cir. Dec. 20, 2024).56 Id. at *5-*6.57 Id. at *7, *17.58 Id. at *10-*11.59 Id. at *12.60 Id. at *15.61 Federal Trade Commission, “Federal Trade Commission Statement Concerning Brand Drug Manufacturers’ Improper Listing of Patents in the Orange Book,” (14 Sept. 2023), https://www.ftc.gov/system/files/ftc_gov/pdf/p239900orangebookpolicystatement092023.pdf.62 Federal Trade Commission, “FTC Challenges More Than 100 Patents as Improperly Listed in the FDA’s Orange Book,” (November 7, 2023), https://www.ftc.gov/news-events/news/press-releases/2023/11/ftc-challenges-more-100-patents-improperly-listed-fdas-orange-book.63 Federal Trade Commission, “FTC Expands Patent Listing Challenges, Targeting More Than 300 Junk Listings for Diabetes, Weight Loss, Asthma and COPD Drugs,” (April 30, 2024), https://www.ftc.gov/news-events/news/press-releases/2024/04/ftc-expands-patent-listing-challenges-targeting-more-300-junk-listings-diabetes-weight-loss-asthma.64 Loper Bright Enterprises v. Raimondo, 603 U.S. 369 (2024).
FDA Proposes New Front-of-Pack Nutrition Label for Packaged Foods
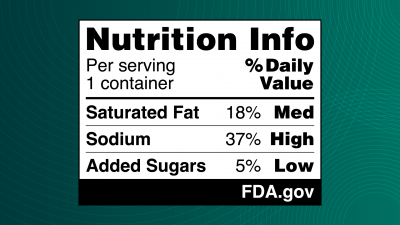
The FDA has announced a proposal to require a new nutrition label on the front of packages for most packaged foods. The label design, shown below, would give consumers readily visible information about a food’s saturated fat, sodium, and added sugars content—three nutrients the FDA states are directly linked with chronic diseases when consumed in excess. The proposed “Nutrition Info box” rates packaged food as being “Low,” “Med,” or “High” in saturated fat, sodium, and added sugars. It was designed after the FDA conducted an experimental study in 2023 of nearly 10,000 U.S. adults to further explore consumer responses to three different types of front-of-pack labels. The new Nutrition Info box is intended to complement the FDA’s existing Nutrition Facts label.
If finalized, the proposed rule would require food manufacturers to add the Nutrition Info box to most packaged food products three years after the final rule’s effective date for businesses with $10 million or more in annual food sales and four years after the final rule’s effective date for businesses with less than $10 million in annual food sales. Comments on the proposed rule can be submitted electronically to http://www.regulations.gov by May 16, 2025.
HHS OCR Settlements: Last Week in Review
During the week of January 6, 2025, the U.S. Department of Health and Human Services’ Office for Civil Rights (“OCR”) entered into resolution agreements and corrective action plans with Elgon Information Systems (“Elgon”), Virtual Private Network Solutions, LLC (“VPN Solutions”) and USR Holdings, LLC (“USR”) for violations of the Health Insurance Portability and Accountability Act of 1996 (“HIPAA”) Security Rule.
The proposed resolutions with Elgon and VPN Solutions are the eighth and ninth ransomware investigation settlements announced by OCR. Elgon is required to pay $80,000 to OCR and will be subject to its monitoring for three years to ensure compliance with HIPAA. VPN Solutions is required to pay $90,000 and will be subject to one year of monitoring. The corrective action plans also lay out certain steps each entity is required to take to resolve potential violations of the HIPAA Privacy and Security Rules.
The proposed resolution with USR, announced on January 8, 2025, stems from a data breach, during which an unauthorized third party/parties were able to access a database containing the electronic protected health information (“ePHI”) of over 2,900 individuals and able to delete ePHI in the database. The resolution agreement requires USR to pay $337,750 to OCR and take steps to resolve potential violations of the HIPAA Privacy and Security Rules. USR will be subject to OCR monitoring for two years to ensure compliance with HIPAA.
Last week’s flurry of settlements is in keeping with a broader trend of OCR Security Rule enforcement activity in the past year. These agreements underscore how it is critical that organizations of all sizes that handle ePHI ensure their compliance with the HIPAA Security Rule, which requires administrative, physical and technical safeguards to ensure the confidentiality, integrity and availability of ePHI.